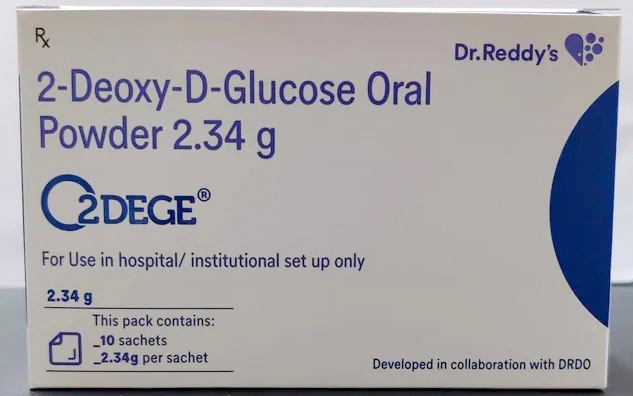
The first batch of the adjunct COVID therapy drug, 2-deoxy-D-glucose (2-DG) which was developed by the Defence Research and Development Organisation (DRDO) in partnership with Dr Reddy’s Laboratories (DRL), Hyderabad, was released on Monday for emergency use.
An anti-COVID-19 therapeutic application of the drug 2-DG has been developed by the Institute of Nuclear Medicine and Allied Sciences (INMAS), a DRDO lab along with DRL.
According to reports, Defence Minister Rajnath Singh formally handed over the drug to Health Minister Dr Harsh Vardhan.
A statement by the Defence Ministry said “One box each of the sachets of the drug was handed over to Dr Randeep Guleria, Director All India Institute of Medical Sciences (AIIMS) and Lt Gen. Sunil Kant of Armed Forces Medical Services (AFMS). More will be handed over to different hospitals across the country for emergency use.”
DRL will ramp up production of the drug which is expected to be made available to all hospitals by the first week of June, said K. Satish Reddy, Chairman DRL
The development is hailed as an example of DRDO and private partnership, which will help patients in overcoming oxygen dependency by around 40%.
Dr Harsh Vardhan said 2-DG was the first therapeutic drug for COVID which India has developed indigenously.
According to DRDO, clinical trial results have shown that this molecule helps in faster recovery of hospitalised patients and reduces supplemental oxygen dependence.
Speaking on the development, DG Satheesh Reddy, Chairman DRDO said, “Scientists have been working on the molecule for long and over the last one-year clinical trials were conducted extensively in various hospitals across the country.”
On May 1, the Drugs Controller General of India (DCGI) granted permission for emergency use of this drug as an adjunct therapy in moderate to severe COVID-19 patients.
Phase-II of the clinical trials of 2-DG in COVID-19 patients was approved by DCGI in May 2020 and Phase-III clinical trials were permitted in November 2020.
According to DRDO, the Phase-III clinical trial was conducted on 220 patients between December 2020 and March 2021 in hospitals in Delhi, Uttar Pradesh, West Bengal, Gujarat, Rajasthan, Maharashtra, Andhra Pradesh, Telangana, Karnataka and Tamil Nadu.